Significant challenges still loom over the oligo industry, from validating novel analytical methods and enhancing the accuracy of existing ones to scaling up manufacturing processes and characterizing complex oligos. As the complexity of oligo therapeutics increases, so does the need for robust, precise, and scalable solutions that can meet stringent regulatory expectations.
With this, the Oligonucleotide Analytical Development & CMC Summit is back in Boston for its 4th year, providing a specialised platform for in-depth discussions on cutting-edge analytical approaches. Expect an in-depth exploration of approaches for impurity profiling through advanced analytical techniques such as HPLC, LC-MS/MS and 31P NMR, a spotlight on the growing role of mechanistic modelling in streamlining chromatography method development, and insights into predictive downscale models to minimise risk in large-scale manufacturing.
From automated RTD modelling in solid phase synthesis to orthogonal approaches for impurity detection and diastereomers ratio monitoring, this summit is the ultimate forum to stay at the forefront of oligo drug development.
What's New for 2025

Gain strategic insight into stereochemical comparability and diastereomer analysis from materials to final drug product by industry pioneers such as Biogen, Novo Nordisk & Wave Life Sciences

Dive into advanced analytical techniques for critical impurity profiling and separation with a case study from Sarepta showcasing the integration of mechanistic models in AEX

Discover how Ionis is revolutionising solid phase synthesis through rapid automated residence time distribution modelling to improve efficiency and minimise waste

Understand evolving regulatory expectations for comparability assessments and how to effectively manage process changes while ensuring consistent critical quality attributes

Explore innovative approaches to oligo characterising from early-stage programs with CAMP4 to advanced purity characterisation using high resolution mass spectrometry from GSK
Who Will You Meet?
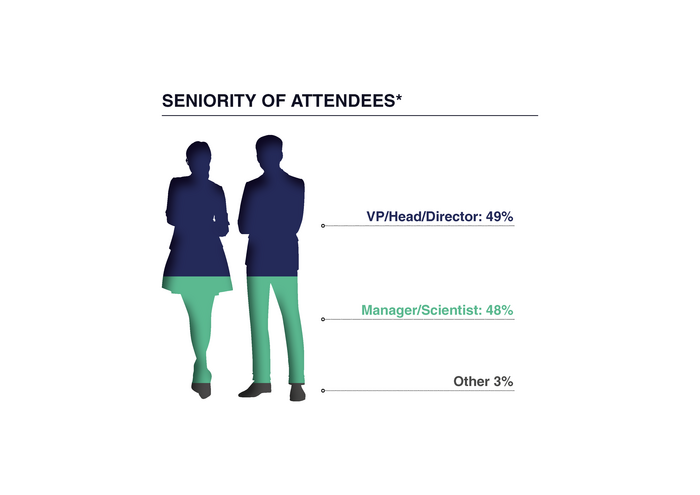
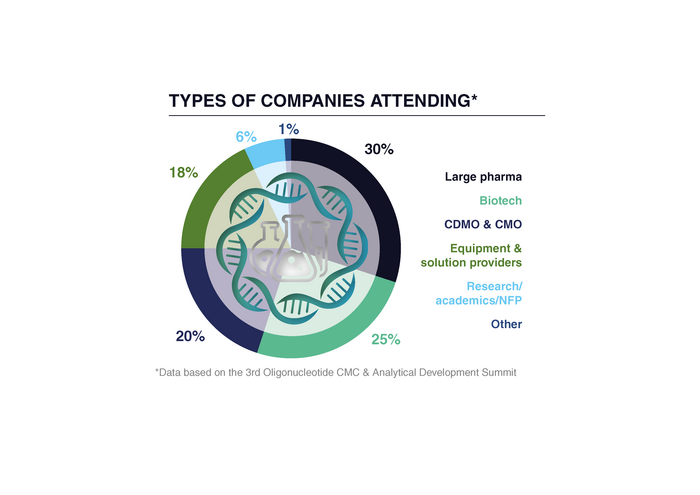
This Summit connects directors, vice presidents, managers and scientists in analytical development, analytical chemistry, CMC, drug product development, process development, and quality control.
What Your Peers Have to Say:
“The networking event was organized to provide excellent opportunities for participants to communicate and exchange information. I thoroughly enjoyed the conversations with other attendees. The quality of the presentations was outstanding.”- Director of Analytical Development at Codexis


“Very intimate conference. Felt connected to the speakers and they were all very approachable and provided excellent dialog.”- Analytical Development Manager at Novo Nordisk
“I am very happy with the number of attendees that attended and the topic of the talks and workshops. I have learned so much about the oligonucleotides in many aspects from these talks and from talking with the attendees and speakers.” - Quality Assurance Manager, Sanofi


“This is my second year attending Oligonucleotide CMC and Analytical Development Summit. The second-year experience validated my observations that it is an excellent opportunity to learn and discuss the current progress in the field.” - Senior Principal Scientist, Vertex

